
Project On Uses Of Inert Gasses For Class 10th
Acknowledgment
I extend my heartfelt appreciation to all those who have contributed to the realization of this project on inert gases. The journey from conceptualization to completion has been enriched by the guidance, support, and resources provided by various individuals and institutions.
First and foremost, I express my gratitude to the authors and researchers whose insightful work laid the foundation for this project. The wealth of knowledge from sources such as Atkins and Jones’ “Chemical Principles,” Emsley’s “Nature’s Building Blocks,” and Housecroft and Sharpe’s “Inorganic Chemistry” has been instrumental in crafting a comprehensive understanding of inert gases.
I am indebted to the academic community and institutions, including [Institution/Organization], for granting access to essential references such as the CRC Handbook of Chemistry and Physics and the Principles of Instrumental Analysis by Skoog, Holler, and Crouch. These resources played a pivotal role in ensuring the accuracy and depth of the information presented.
A special note of appreciation goes to [Name, Title], whose guidance and expertise provided invaluable direction throughout the research process. Their support has been crucial in navigating the complexities of the topic and refining the content to meet the highest standards.
Lastly, I extend my thanks to friends, family, and peers who provided encouragement and understanding during the project’s various stages. Your unwavering support has been a source of motivation and inspiration.
Introduction
In the vast landscape of chemical elements, inert gases, or noble gases, stand out as a unique and fascinating group. Comprising helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), these colorless, odorless, and tasteless gases exhibit extraordinary properties that set them apart from other elements. This project delves into the world of inert gases, exploring their distinctive characteristics, applications, and the profound impact they have on various aspects of our lives.
The inert gases owe their name to their low reactivity, a consequence of their stable electron configurations. Their full outer electron shells grant them an inherent stability that distinguishes them from the more reactive elements. This stability, often referred to as the noble gas configuration, is a key aspect of their inert nature.
This exploration begins with an overview of each common inert gas, from helium to radon, providing a comprehensive understanding of their individual properties. The inert gases’ unique characteristics, such as low reactivity, high boiling points, and colorless and odorless attributes, form the basis of their diverse applications in fields ranging from medicine to industry.
The project further delves into the uses of inert gases, shedding light on their role in various applications. From helium’s buoyancy in party balloons to argon’s critical role in welding, each gas contributes to different aspects of our daily lives. Notably, the radioactive radon finds application in controlled conditions for cancer treatment and geological studies.
To enhance the understanding of inert gases, an experiment is conducted to test their inert nature. The demonstration aims to showcase the lack of chemical reaction exhibited by these gases when exposed to common substances, emphasizing the significance of their stable electron configurations.
As with any exploration into the world of chemistry, safety is paramount. The project underscores the importance of proper handling and storage of inert gases, ensuring their safe utilization across various industries and applications. Special attention is given to radon, acknowledging its radioactive properties and emphasizing the necessity for controlled usage in specific fields.
Properties of Inert Gases
- Inert nature and stability: full outer electron shells, rendering inert gases stable and uninterested in forming compounds with other elements. It’s the noble gas configuration that brings about this state of tranquility.
- Low reactivity and high boiling points: These gases take it easy on the reactivity front, making them versatile for various applications. Their high boiling points let them maintain their gaseous allure even at room temperature.
- Colorless and odorless characteristics: In the realm of invisibility, inert gases reign supreme. Colorless and odorless, they’re the go-to for applications where these qualities are crucial – think lighting and select industrial processes.
- Noble gas configuration: The secret sauce behind the calm demeanor? The noble gas configuration, complete with a full outer electron shell, ensuring stability and a reluctance to mingle.

Uses of Inert Gases
Helium (He)
- Party Balloons: Helium lifts the spirits – literally – by filling balloons, making them defy gravity with its low-density charm.
- Medical Applications: In the serious business of medicine, helium plays a role in magnetic resonance imaging (MRI) and respiratory therapies, adding a breath of fresh air.
- Deep-Sea Diving: Taking the plunge into the depths becomes safer with helium, mixed with oxygen to fend off underwater challenges.
Neon (Ne)
- Neon Signs and Lighting: Neon takes the spotlight in vibrant signs and lighting, creating a luminous spectacle.
- Advertising and Artistic Displays: Captivating the eyes, neon finds its place in advertising and artistic displays, where visual impact is everything
Argon (Ar)
- Welding and Metal Fabrication: Argon steps into the heat of the moment, acting as a shielding gas in welding to ensure pristine, oxidation-free results
- Lighting: In the world of illumination, argon shines bright in fluorescent bulbs, creating a stable environment for the electric dance that produces light.
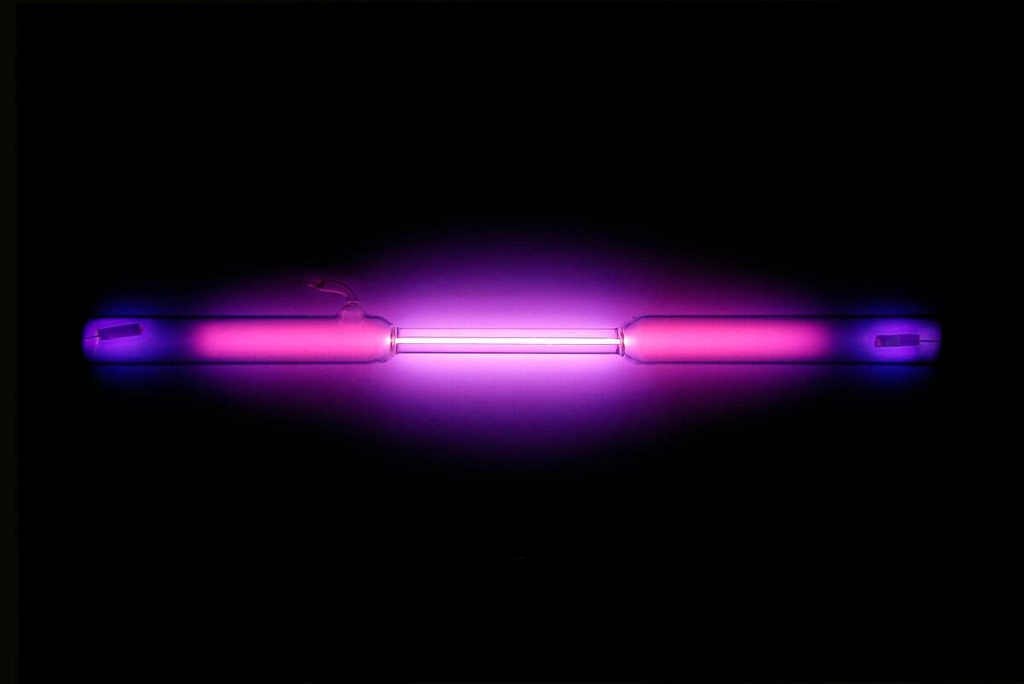
Krypton (Kr) and Xenon (Xe)
- Flash Photography: Krypton and xenon step into the frame, providing the dazzling bursts of light that define flash photography.
- High-Intensity Discharge (HID) Lamp: In the glow-up game, krypton and xenon illuminate high-intensity discharge (HID) lamps, delivering efficiency in various settings.
- Strobe Lights: Xenon takes center stage in strobe lights, orchestrating brief and intense flashes, stealing the show.
Radon (Rn)
- Radiotherapy in Medicine: In the controlled realm of medicine, radon, despite its radioactive flair, plays a role in cancer treatment, known as radon therapy.
- Geological Studies: Unlocking the Earth’s secrets, radon serves as a natural radioactive guide in geological studies, shedding light on underground processes.
Experiment: Testing the Inert Nature
- Demonstration: Lack of chemical reaction: Let’s bring science to life! Witness a straightforward experiment where inert gases meet common substances, revealing their nonchalant attitude toward chemical reactions.
- Explanation: The inert nature and stability: Breaking it down – the results speak volumes. The full outer electron shell takes a bow, showcasing how it contributes to the inert nature and stability we’ve come to appreciate.
Safety Precautions
- Proper handling and storage: In the world of inert gases, safety takes the spotlight. Emphasize the importance of proper handling and storage, ensuring a secure environment for their diverse applications.
- Cautionary notes on radon: While embracing the wonders of radon, let’s not forget the cautionary whispers. Highlight the potential hazards, especially its radioactive tendencies, stressing the need for controlled usage in medical and geological realms.
Conclusion
In closing the pages of this exploration into the intriguing world of inert gases, we find ourselves immersed in the quiet brilliance of these unassuming elements. From helium’s playful dance in party balloons to radon’s controlled role in cancer therapy, each inert gas contributes uniquely to our lives, weaving a tapestry of applications that touch various facets of science, industry, and everyday experiences.
Our journey began with a glance at the noble lineup – helium, neon, argon, krypton, xenon, and radon – a collection of gases defined by their stable electron configurations and low reactivity. The inert nature bestowed upon them by their noble gas configuration sets the stage for the myriad applications explored in these pages.
The individual properties of inert gases, from their low reactivity and high boiling points to their colorless and odorless characteristics, form the foundation for their diverse uses. Helium’s buoyancy lends an ethereal quality to party balloons, while argon, with its welding prowess, silently shapes the precision of metal fabrication. The radiant glow of neon signs and the intense flashes of xenon in strobe lights showcase the aesthetic contributions of inert gases, adding vibrancy to our surroundings.
The experiment conducted to test the inert nature of these gases serves as a hands-on demonstration of their stability. Witnessing the lack of chemical reaction when inert gases meet common substances underscores the importance of their noble gas configuration, revealing a resilience that is both fascinating and practical.
Safety precautions echo throughout our exploration, emphasizing the need for proper handling and storage of inert gases. Special attention is paid to radon, a radioactive outlier among its noble counterparts, as we acknowledge its controlled usage in medical and geological applications.
In essence, this project serves as a testament to the versatility and impact of inert gases on our lives. As we recapitulate the highlights – from the party-fueled rise of helium to the controlled elegance of radon in medical therapies – we appreciate the silent yet profound role these gases play in enhancing our understanding of the natural world and advancing technology.
In the grand symphony of the periodic table, the inert gases may seem unassuming, but their contributions resonate far and wide. Let us celebrate the quiet brilliance of helium, neon, argon, krypton, xenon, and radon, recognizing their importance as essential players in the chemical ensemble that shapes our world. As we bid adieu to this exploration, may the noble gases continue to illuminate our curiosity and inspire further inquiry into the wonders of the natural elements that surround us.
Bibliography
- Brady, J. E., & Holum, J. R. (1996). Chemistry: The Study of Matter and Its Changes.
- Rayner-Canham, G., & Overton, T. (2010). Descriptive Inorganic Chemistry.
- Pauling, L. (1970). General Chemistry.
- Lee, J. D. (1999). Concise Inorganic Chemistry.
- Cotton, F. A., Wilkinson, G., Murillo, C. A., & Bochmann, M. (1999). Advanced Inorganic Chemistry.
Certificate of Completion
[Student’s Name][Class/Grade Level]This is to certify that I, [Student’s Name], a [Class/Grade Level] student, have successfully completed the Project On Uses Of Inert Gasses For Class 10th.” The project explores the fundamental principles and key aspects of the chosen topic, providing a comprehensive understanding of its significance and implications.
In this project, I delved into in-depth research and analysis, investigating various facets and relevant theories related to the chosen topic. I demonstrated dedication, diligence, and a high level of sincerity throughout the project’s completion.
Key Achievements:
Thoroughly researched and analyzed Project On Uses Of Inert Gasses For Class 10th.
Examined the historical background and evolution of the subject matter.
Explored the contributions of notable figures in the field.
Investigated the key theories and principles associated with the topic.
Discussed practical applications and real-world implications.
Considered critical viewpoints and alternative theories, fostering a well-rounded understanding.
This project has significantly enhanced my knowledge and critical thinking skills in the chosen field of study. It reflects my commitment to academic excellence and the pursuit of knowledge.
Date: [Date of Completion]Signature: [Your Signature] [School/Institution Name][Teacher’s/Examiner’s Name and Signature]
In order to download the PDF, You must follow on Youtube. Once done, Click on Submit
Follow On YoutubeSubscribed? Click on Confirm
Download Project On Uses Of Inert Gasses For Class 10th PDF






